
AFWG shall not bear any responsibility for any content on such sites. Any link to a third-party site does not constitute an endorsement of the third party, their site or services. AFWG also makes no warranties as to the content of such sites.
Would you like to continue?
Professor Ritesh Agarwal1, Professor Arunaloke Chakrabarti2
1Additional Professor, Department of Pulmonary Medicine,
2Professor, Department of Medical Microbiology,
Post Graduate Institute of Medical Education and Research, Chandigarh, India
Asthma is a chronic inflammatory disease of the airways characterized by bronchial hyperresponsiveness and variable airflow obstruction. It manifests clinically as recurrent episodes of wheezing, breathlessness, chest tightness and cough.1 There is current evidence to demonstrate a close association between fungi and asthma, the so-called fungal asthma or allergic fungal airway disease.2,3 Fungi can colonize the tracheobronchial tree of asthmatic patients with subsequent release of fungal antigens, which can trigger or worsen asthma. Several small studies have identified certain genetic factors linking the risk of fungal asthma with individual mutations in mediators of innate and adaptive immunity.4,5 Fungal asthma can manifest itself as fungal sensitization or fungal allergy. Fungal sensitization is an immune-mediated response to a fungus, without evidence of inflammation or tissue damage, clinically documented by an elevated fungal-specific IgE. Fungal allergy is an immune-mediated inflammatory response to a fungus causing tissue damage.
Table. Clinical manifestations of fungal asthma (in increasing order of severity) |
| Aspergillus fumigatus-associated asthma (AFAA) |
| Controlled asthma Elevated A. fumigatus (or other fungus) specific IgE (>0.35 kUA/L) Total IgE <500 IU/mL |
| Severe asthma with fungal sensitization (SAFS) |
| Severe asthma Elevated A. fumigatus (or other fungus) specific IgE (>0.35 kUA/L) Total IgE 500-1000 IU/mL Normal A. fumigatus specific IgG (<27 mgA/L) |
| Serologic allergic bronchopulmonary ABPA (ABPA-S) |
| Asthma A. fumigatus specific IgE >0.35 kUA/L Total IgE >1000 IU/mL Elevated A. fumigatus specific IgG (>27 mgA/L) Transient or fixed pulmonary opacities Total eosinophil count >500 cells/μL Normal high-resolution CT of the chest |
| ABPA with bronchiectasis (ABPA-B) |
| Asthma A. fumigatus specific IgE >0.35 kUA/L Total IgE >1000 IU/mL Elevated A. fumigatus specific IgG (>27 mgA/L) Transient or fixed pulmonary opacities Total eosinophil count >500 cells/μL High-resolution CT of the chest showing bronchiectasis |
Although several fungi can cause fungal sensitization, fungal allergy is caused only by a small number of thermotolerant fungi, the most common being Aspergillus fumigatus.6-8 The clinical presentation of fungal asthma can vary from fungal sensitization at one end, manifesting as Aspergillus fumigatus-associated asthma (AFAA) to ABPA at the most extreme end of the spectrum (Table). Severe asthma with fungal sensitization (SAFS) defines a phenotype of severe asthma characterized by severe asthma and fungal sensitization but exclusion of ABPA.2,7 ABPA is the most well-characterized form of fungal asthma that manifests clinically as difficult-to-control asthma, recurrent pulmonary opacities and bronchiectasis (Figure).9 The interest in ABPA stems from the fact that if the disorder is recognized and treated adequately, the onset and/or progression of the irreversible manifestations of ABPA including bronchiectasis and pulmonary fibrosis can be halted.
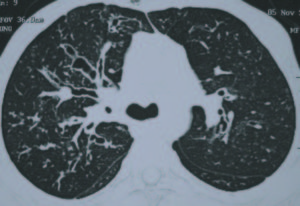
The diagnosis of fungal asthma is made on a combination of clinical, radiological and immunological findings (Table). The most useful investigations in differentiating between various entities of fungal asthma include total IgE and A. fumigatus specific IgG levels. It is important to differentiate between these various entities as the treatment options are different. The treatment principles in fungal asthma include avoidance of fungal exposure, control of the underlying inflammatory process with glucocorticoids and attenuating fungal burden with the use of antifungal therapies. While patients with AFAA do not require any particular treatment, patients with SAFS may benefit from itraconazole therapy (400 mg/day for six months).10 On the other hand, the initial treatment of choice for ABPA is glucocorticoids.11 Unfortunately, the natural history of ABPA is characterized by recurrent episodes of exacerbation requiring repeated treatment with glucocorticoids.12 In such situations, antifungal treatment with itraconazole can serve as steroid-sparing agent.13,14 In those with recurrent exacerbations, the use of nebulized amphotericin B has been shown to reduce the frequency of exacerbations.15,16 Other treatment options include newer azoles such as voriconazole and posaconazole, omalizumab and methylprednisolone pulses.
Before initiating glucocorticoids, physicians should carefully evaluate the potential risk and benefit of the use of glucocorticoids in an individual patient due to the following concerns. First, glucocorticoids enhance the growth rate of A. fumigatus and A. flavus in vitro.17 Hypercortisolemic patients, such as those with endogenous Cushing’s syndrome, and much more frequently, those receiving exogenous glucocorticoids, are especially at risk of invasive fungal diseases. This vulnerability is attributed to the complex dysregulation of immunity caused by glucocorticoids.18
Finally, several issues remain unsolved. There is still no clear consensus or guideline to suggest when and in whom should an antifungal agent be given in addition to glucocorticoids. Also, there is still uncertainty regarding the fungi that are relevant in fungal asthma, the natural history of fungal sensitization and its relationship with fungal allergy, and the pathogenesis and treatment of fungal asthma. Clearly, more data is required in asthmatic patients to determine the relationship between fungal sensitization and the development or progression of lung damage, as well as the clinical and immunological criteria needed for the definition of AFAA, SAFS and ABPA.