
AFWG shall not bear any responsibility for any content on such sites. Any link to a third-party site does not constitute an endorsement of the third party, their site or services. AFWG also makes no warranties as to the content of such sites.
Would you like to continue?
Dr Methee Chayakulkeeree
Associate Professor
Division of Infectious Disease and Tropical Medicine
Department of Medicine
Faculty of Medicine, Siriraj Hospital
Mahidol University
Bangkok, Thailand
Studies have demonstrated the benefit of antifungal prophylaxis in high-risk patients with hematologic diseases. For instance, posaconazole was shown to prevent invasive fungal diseases (IFD) significantly more effectively than fluconazole or itraconazole, and improved overall survival in neutropenic patients undergoing chemotherapy for acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS).1 Posaconazole, voriconazole, fluconazole and itraconazole were similarly found to be beneficial as antifungal prophylaxis in high-risk patients after allogeneic hematopoietic stem cell transplantation (HSCT).2-4 In HSCT recipients, although these agents showed efficacy to prevent IFD, they did not seem to have survival benefit according to several clinical studies. Data regarding cost-effectiveness of antifungal prophylaxis in patients with hematologic diseases or after HSCT in resource-limited settings or developing countries are lacking and should be considered before universal implementation.
Recent data have also shown that new treatment strategies in chronic lymphoproliferative disorders (CLD) have increased the risk of IFD.5 Given these findings, patients with CLD could possibly be considered eligible for antifungal prophylaxis. However, drug-drug interactions between azoles and vincristine are a major obstacle in this issue. More data are needed before generalized recommendations can be made.
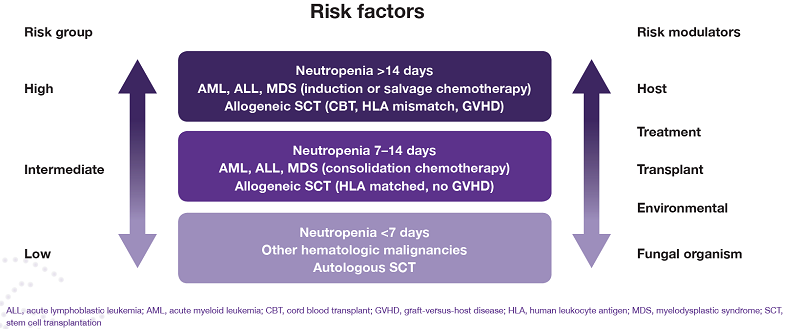
The underlying disease is not the only factor that places a patient at high risk of IFD; risk modulators that should also be considered include transplant-related factors, environmental factors, treatment and even the organism itself (Figure).6
Figure. Risk groups for invasive fungal infections6
Dr Chayakulkeeree discussed considerations for using antifungal prophylaxis:
Pre-emptive antifungal therapy, based on galactomannan enzyme immunoassay and high-resolution computed tomography (HRCT), has been shown to reduce the rate of antifungal use and yielded a 12-week survival rate of 63.6% for patients with IFD.9
In another trial that compared empirical versus pre-emptive antifungal therapy for high-risk, febrile neutropenic patients, pre-emptive treatment decreased treatment costs by 35%, but was associated with more IFD than empirical treatment. Survival rates were similar between the two groups.10
Some limitations of the pre-emptive diagnosis-drivenantifungal approach must be noted: availability of the galactomannan assay (twice a week); availability of HRCT within 24 hours when requested; false-positive and false-negative galactomannan results; and a biomarker for candidiasis may need to be included.
The León score11 and the Ostrosky-Zeichner score12 were designed to identify patients at high risk of invasive candidiasis in the ICU. However, both these scores are not highly sensitive and may not be reliable predictors of invasive candidiasis.
In the EMPIRICUS study of patients with ICU-acquired sepsis, Candida colonization and multiple organ failure, empirical treatment with micafungin did not increase fungal infection-free survival compared with placebo.13 As such, empirical antifungal treatment in high-risk ICU patients cannot be strongly recommended at this time.14 In some circumstances, for patients’ safety, empirical antifungal treatment in high-risk ICU patients with sepsis may be considered but need to be modified or discontinued upon availability of fungal culture and susceptibility results. Therefore, development of rapid and accurate non–culture-based diagnostic tests is crucial to facilitate appropriate empirical antifungal treatment in ICU patients.
The combination of amphotericin B and flucytosine was shown to be effective as induction therapy for HIV-associated cryptococcal meningitis, and was noninferior to 2 weeks of amphotericin B-based therapy.15
However, combination antifungal therapy is still controversial in other fungal infections, such as invasive candidiasis, invasive aspergillosis and mucormycosis, and more data are needed to demonstrate the clear role of combination antifungal therapy in these infections.16
References